IJCRR - 7(2), January, 2015
Pages: 47-53
Print Article
Download XML Download PDF
NISIN: PRODUCTION AND MECHANISM OF ANTIMICROBIAL ACTION
Author: Sukrita Punyauppa-path, Parichat Phumkhachorn, Pongsak Rattanachaikunsopon
Category: Healthcare
Abstract:Nisin is a heat stable lantibiotic consisting of 34 amino acids. Of these amino acids, there are several unusual amino acids including dehydroalanine, dehydrobutyrine, aminobutyric acid, lanthionine and \?-methyllanthionine. It has antimicrobial activity against many species of Gram positive bacteria, but not Gram negative bacteria due to their outer membrane barriers. However, when used in combination with other chemical or physical treatments that destabilize the outer membranes, nisin can inhibit Gram negative bacteria. Nisin has been used as a food preservative in many food industries because it is legally approved as safe for use in food and beverage. The knowledge on the production and mechanism of antimicrobial action of nisin is important for the understanding how nisin contains unusual amino acids and how it kills sensitive bacteria. The knowledge may also be a factor for the successful application of nisin. Therefore, this review focuses on presenting these two aspects of nisin.
Keywords: Bacteriocin, Lactococcus lactis, lanbiotic, nisin
Full Text:
INTRODUCTION
Nisin is the antimcirobial peptide produced by Lactococcus lactis subsp. Lactis1 . It is the only bacterioicin that have been legally approved as safe for use in food and beverage. Nisin was first commercially marketed in England in 1953. In 1969, a joint commission between the Food and Agriculture Organization of the United Nation (FAO) and The World Health Organization (WHO) recognized nisin as a safe and legal biological food preservative. In the United States, the use of nisin in food has been legally approved by the American Food and Drug Administration (FDA) since 19882 . The word “nisin” (Group N Inhibitory Substance + the suffix “in”) was coined by Mattick and Hirsch3 to distinguish nisin from the bacteriocin produced by Lactococcus lactis subsp. cremoris called diplococcin. Nisin is a heat stable, cationic lantibiotic belonging to class I bacteriocin according to the classification criteria of Klaenhammer4 . It consists of 34 amino acids. Of these amino acids, there are several unusual amino acids including dehydroalanine (Dha), dehydrobutyrine (Dhb), aminobutyric acid (Aba), lanthionine (Ala-S-Ala) and β-methyllanthionine (Aba-S-Ala). Nisin has antimicrobial activity against many species of Gram positive bacteria (Table 1), but not Gram negative bacteria due to their outer membrane barrier. Normally, nisin producer has immunity to its own produced nisin but not to other lantibotics. This is for protecting itself from being killed by its own nisin. Although it is a protein, it is not digested by all of the protein digesting enzymes. It is sensitive to chymotrypsin, but not to trypsin and pronase5 . At present, several natural nisin variants have been reported including nisin A1 , nisin Z6 , nisin Q7 , nisin F8 , nisin U8 and nisin U28 . Nisin A, nisin Z, nisin Q and nisin F are produced by Lactococcous lactis while nisin U and nisin U2 produced by Streptococcus sp.8 Of the bacteriocins, only nisin A and nisin Z have been extensively studied. These variants differ in a single amino acid residue at position 27 which is histidine in nisin A and aspartic acid in nisin Z.
PRODUCTION AND MODIFICATION OF NISIN
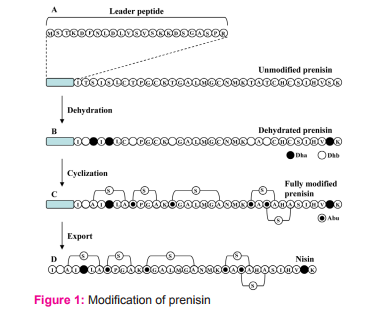
Nisin is ribosomally produced from a structural gene as a prenisin having 57 amino acids (Fig. 1 A). It is an inactive form of nisin containing a leader peptide (having 23 amino acids) at the N terminus of the molecule. Modifications after nisin synthesis are dehydration (Fig. 1 B), cyclization (Fig. 1 C) and leader peptide digestion (Fig. 1 D). In the dehydration step, serine and threonine are dehydrated to dehydroalanine (Dha) and dehydrobutyrine (Dhb), respectively. In cyclization step, several thioether crosslinks (S) are formed between alanine and alanine and between aminobutyric acid and alanine. The thioether crosslink between alanine and alanine results in the formation of lanthionine (A-S-A) and that between aminobutyric acid (Abu) and alanine results in the formation of β-methyllanthionine (Abu-S-A). Upon export of nisin outside the cell, leader peptide is digested from the prenisin resulting in the active nisin that is containing 34 amino acids.
MECHANISM OF ANTIMICROBIAL ACTION OF NISIN
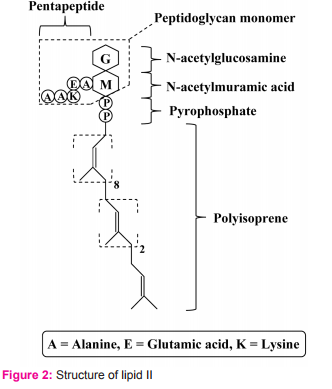
For its killing activity, nisin does not require a membrane receptor on its target cell. This is unlike killing activity of many bacteriocins such as colicin that need membrane receptors on target cells. There are two major steps for nisin to kill sensitive cells. 1. Passage through cell wall To pass through target cell wall, nisin generally interactions (via hydrophobic or electrostatic interactions) with anionic components in the cell wall of sensitive cells such as teichoic acids, teichuronic acids and lipoteichoic acids, acidic polysaccharides or phospholipids9 . 2. Interaction with lipid II Lipid II (Fig. 2) is a membrane anchored cell wall precursor that is essential for bacterial cell wall biosynthesis. It is composed of a membrane anchor of 11 polyisoprene residues to which, via a pyrophosphate, the basic building block of the cell wall (peptidoglycan monomer), Nacetylglucosamine-N-acetylmuramic acid(pentapeptide), is attached. It brings the peptidoglycan monomer from cytoplasm of the bacterial cell to incorporate into growing peptidoglycan network in the bacterial cell wall (Fig. 3).
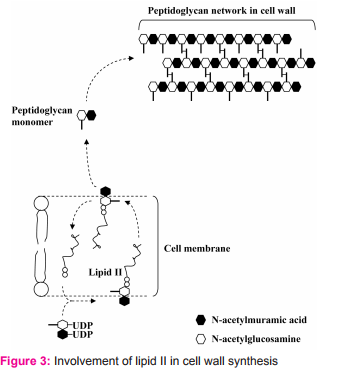
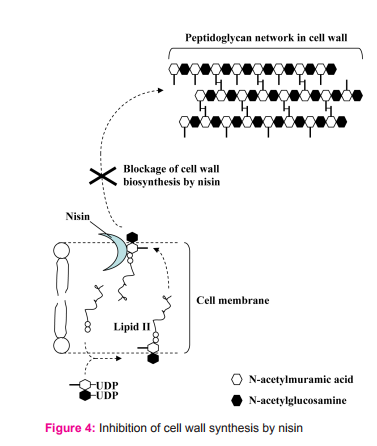
Once nisin reaches cell membrane of the sensitive cells, it may perform one of these actions. 2.1. It binds to lipid II and prevents the peptidoglycan monomer to incorporation into the growing peptidoglycan network (Fig. 4).
2.2. It uses N-terminal binding motif to bind to the carbohydrate-pyrophosphate moiety of lipid II. This enables the C-terminal segment of nisin to insert into the cell membrane. Several nisin-lipid II complexes assemble to form a stable pore with diameter of 2 nanometers in cell membrane of target cells (Fig. 5)10. However, some reports state that the pore is formed by 4 nisin-lipid II complex and 4 nisin molecules (Fig. 6). Once the pore is formed in cell membrane, it can cause an increase in membrane permeability which can lead to the dissipation of the membrane potential, an efflux of small cytoplasmic contents such as amino acids, nucleotides and ions from the damaged cells11,12. Consequently, the damaged cells cannot produce energy as well as vital macromolecules resulting in cell death eventually11,13.
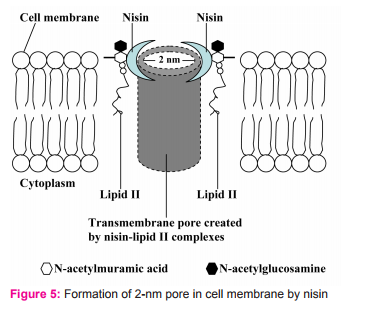
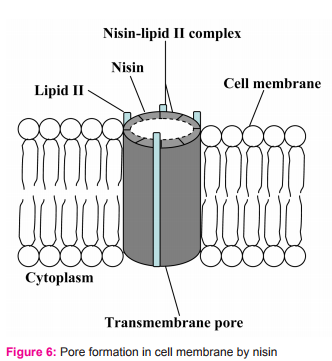
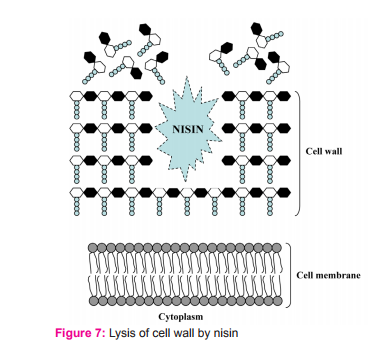
Besides the lipid II mediated mode of action, nisin can cause lysis of the cell wall of sensitive cells (Fig. 7), particularly in staphylococci.

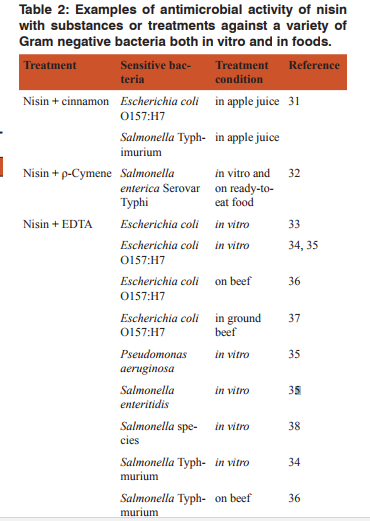
Inhibition of Gram negative bacteria by nisin together with other treatments As mentioned earlier, nisin has antimicrobial activity against some Gram positive bacteria but not against Gram negative bacteria because of their outer membrane barriers. However, many research works show that nisin can inhibit Gram negative bacteria when it is used in combination with substances or physical treatments that destabilize their outer membranes. Table 2 shows some examples of antimicrobial activity of nisin together with other substances or treatments against a variety of Gram negative bacteria both in vitro and in foods.



CONCLUSION
Although the production and posttranslational modification of Nisin are now fully understood, its mechanism of antimicrobial action still requires further investigation. This may be because nisin has more than one mechanisms of antimicrobial action depending on several factors such as structural properties of target bacteria. Nisin, by itself, is only active against Gram positive bacteria. However, the use of Nisin together with outer membrane destabilizing treatments makes it become active against Gram negative bacteria. This finding broadens the application of Nisin as a food preservative
. ACKNOWLEDGEMENT
Authors acknowledge the immense help received from the scholars whose articles are cited and included in references of this manuscript. The authors are also grateful to authors / editors /publishers of all those articles, journals and books from where the literature for this article has been reviewed and discussed.
Source of funding: The Faculty of Science, Ubon Ratchathani University, Thailand
Conflict of Interest: The authors declare that there is no conflict of interest regarding the publication of this article
References:
1. Gross E and Morell JL. The structure of nisin. J Am Chem Soc 1971; 93(18):4634-5.
2. Zacharof MP and Lovittb RW. Bacteriocins produced by lactic acid bacteria a review article. APCBEE Procedia 2012; 2: 50-6.
3. Mattick AT, Hirsch A and Berridge NJ. Further observations on an inhibitory substance (nisin) from lactic streptococci. The Lancet 1947; 250(6462): 5-8.
4. Klaenhammer TR. Genetics of bacteriocins produced by lactic acid bacteria. FEMS Microbiol Rev 1993; 12(1-3): 39-85.
5. Harris LJ, Fleming HP and Klaenhammer TR. Characterization of two nisin-producing Lactococcus lactis subsp. lactis strains isolated from a commercial sauerkraut fermentation. Appl Environ Microbiol 1992; 58(2): 1477-83.
6. Mulders JW, Boerrigter IJ, Rollema HS, Siezen RJ and de Vos WM. Identification and characterization of the lantibiotic nisin Z, a natural nisin variant. Eur J Biochem 1991; 201(3): 581-4.
7. Yoneyama F, Fukao M, Zendo T, Nakayama J and Sonomoto K. Biosynthetic characterization and biochemical features of the third natural nisin variant, nisin Q, produced by Lactococcus lactis 61-14. J Appl Microbiol 2008; 105(6): 1982-90
. 8. Piper C, Hill C, Cotter PD and Ross RP. Bioengineering of a Nisin A-producing Lactococcus lactis to create isogenic strains producing the natural variants Nisin F, Q and Z. Microb Biotechnol 2011; 4(3): 375-82.
9. Kramer NE, Hasper HE, van den Bogaard PT, Morath S, de Kruijff B, Hartung T, Smid EJ, Breukink E, Kok J and Kuipers OP. Increased D-alanylation of lipoteichoic acid and a thickened septum are main determinants in the nisin resistance mechanism of Lactococcus lactis. Microbiology 2008; 154: 1755-62.
10. Wiedemann I, Benz R and Sahl HG. Lipid II-mediated pore formation by the peptide antibiotic nisin: a black lipid membrane study. J Bacteriol 2004; 186(10): 3259-61.
11. Sahl HG, Kordel M and Benz R. Voltage-dependent depolarization of bacterial membranes and artificial lipid bilayers by the peptide antibiotic nisin. Arch Microbiol 1987; 149(2): 120-4.
12. Schuller F, Benz R and Sahl HG. The peptide antibiotic subtilin acts by formation of voltage-dependent multi-stateores in bacterial and artificial membranes. Eur J Biochem 1989; 182(1): 181-6.
13. Ruhr E and Sahl HG. Mode of action of the peptide antibiotic nisin and influence on the membrane potential of whole cells and on cytoplasmic and artificial membrane vesicles. Antimicrob Agents Chemother 1985; 27(5): 841- 5.
14. Komitopoulou E, Boziaris IS, Davies EA, Delves-Broughton J and Adams MA. Alicyclobacillus acidoterrestris in fruit juices and its control by nisin. Int J Food Microbiol 1999; 34(1): 81-5.
15. Pol IE, Mastwijk HC, Bartels PV and Smid EJ. Pulsed-electric field treatment enhances the bactericidal action of nisin against Bacillus cereus. Appl Environ Microbiol 2000; 66 (1): 428-30.
16. Alrabadi NI. Shelf life extension of cheddar processed cheese using polyethylene coating films of nisin against Bacillus cereus. J Biol Sci 2012; 12(7): 406-10.
17. Li T, Tao J and Hong F. Study on the inhibition effect of nisin. J Am Sci 2005; 1(2): 33-7.
18. Scott V and Taylor S. Effect of Nisin on the Outgrowth of Clostridium botulinum Spores. J Food Sci 1981; 46(1): 117-26.
19. Kerr KG, Copley RM and Wilcox MH. Activity of nisin against Clostridium difficile. The Lancet 1997; 349(9057): 1026-7.
20. Rattanachaikunsopon P and Phumkhachorn P. Characterization of nisin produced by Lactococcus lactis RP359 isolated from Kem-Buk-Nud, a traditional Thai fermented food. Internet J Microbiol 2008; 5(1).
21. Benkerroum N and Sandine WE. Inhibitory action of nisin against Listeria monocytogenes. J Dairy Sci 1988; 71(2): 3237-45.
22. Chung KT, Dickson JS and Crouse JD. Effects of nisin on growth of bacteria attached to meat. Appl Environ Microbiol 1989; 55(6):1329-33.
23. Dong-Sun J, Bodyfelt FW and Daeschel MA., Influence of fat and emulsifiers on the efficacy of nisin in inhibiting Listeria monocytogenes in fluid milk. J Dairy Sci 1992; 75(2): 387-93.
24. Coma V, Sebti I, Pardon P, Deschamps A and Pichavant FH. Antimicrobial edible packaging based on cellulosic ethers, fatty acids, and nisin incorporation to inhibit Listeria innocua and Staphylococcus aureus. J Food Prot 2001; 64(4): 470-5.
25. Calderon-Miranda ML, Barbosa-Canovas GV and Swanson BG. Inactivation of Listeria innocua in liquid whole egg by pulsed electric fields and nisin. Int J Food Microbiol 1999; 51(1): 7-17.
26. Dutreux N, Notermans S, Gongora-Nieto MM, BarbosaCanovas GV and Swanson BG. Effects of combined exposure of Micrococcus luteus to nisin and pulsed electric fields. Int J Food Microbiol 2000; 60(2-3): 147-52.
27. Mauriello G, De Luca E, La Storia A, Villani F and Ercolini D. Antimicrobial activity of a nisin-activated plastic film for food packaging. Lett Appl Microbiol 2005; 41(6): 464-9.
28. Sobrino-Lopez A and Martin Belloso O. Enhancing inactivation of Staphylococcus aureus in skim milk by combining high-intensity pulsed electric fields and nisin. J Food Prot 2006; 69(2): 345-53.
29. Goldstein BP, Wei J, Greenberg K and Novick R. Activity of nisin against Streptococcus pneumoniae, in vitro, and in a mouse infection model. J Antimicrob Chemother 1998; 42(2): 277-8.
30. Lebel G, Piche F, Frenette M, Gottschalk M and Grenier D. Antimicrobial activity of nisin against the swine pathogen Streptococcus suis and its synergistic interaction with antibiotics. Peptides 2013; 50: 19-23.
31. Yuste J and Fung DY. Inactivation of Salmonella typhimurium and Escherichia coli O157:H7 in apple juice by a combination of nisin and cinnamon. J Food Prot 2004; 67(2): 371-7.
32. Rattanachaikunsopon P and Phumkhachorn P. Synergistic antimicrobial effect of nisin and ρ-cymene on Salmonella enterica serovar Typhi in vitro and ready-to-eat food. Biosci Biotechnol Biochem 2010; 74(3): 520-4
33. Schved F, Henis Y and Juven BJ. Response of spheroplasts and chelator-permeabilized cells of Gram-negative bacteria to the action of the bacteriocins pediocin SJ-1 and nisin. Int J Food Microbiol 1994; 21(4): 305-14.
34. Cutter CN and Siragusa GR. Population reductions of Gram-negative pathogens following treatments with nisin and chelators under various conditions. J Food Prot 1995; 58(9):977-83.
35. Boziaris IS and Adams MR. Effect of chelators and nisin produced in situ on inhibition and inactivation of Gram negatives. Int J Food Microbiol 1999; 53(2-3): 105-13.
36. Cutter CN and Siragusa GR. Treatments with nisin and chelators to reduce Salmonella and Escherichia coli on beef. J Food Prot 1995; 58(9): 1028-30.
37. Fang TJ and Tsai HC. Growth patterns of Escherichia coli O157:H7 in ground beef treated with nisin, chelators, organic acids and their combinations immobilized in calcium alginate gels. Food Microbiol 2003; 20: 243-53.
38. Stevens KA, Sheldon BW, Klapes NA and Klaenhammer TR. Nisin treatment for inactivation of Salmonella species and other Gram-negative bacteria. Appl Environ Microbiol 1991; 57(12): 3613-5.
39. Lee JI, Lee HJ and Lee MH. Synergistic effect of nisin and heat treatment on the growth of Escherichia coli O157:H7. J Food Prot 2002; 65(2): 408-10.
40. Boziaris IS, Humpheson L and Adams MR. Effect of nisin on heat injury and inactivation of Salmonella enteritidis PT4. Int J Food Microbiol 1998; 43(1-2): 7-13.
41. Esteban MD, Aznar A, Fernandez PS and Palop A. Combined effect of nisin, carvacrol and a previous thermal treatment on the growth of Salmonella enteritidis and Salmonella senftenberg. Food Sci Technol Int 2013; 19(4): 357-64.
42. Hauben KJA, Wuytack EY, Soontjens CCF and Michiels CW. High-pressure transient sensitization of Escherichia coli to nisin and lysozyme by disruption of outer-membrane permeability. J Food Prot 1996; 59(4): 350-5.
43. Masschalck B, Garcia-Graells C, Van Haver E and Michiels C. Inactivation of high pressure resistant Escherichia coli by lysozyme and nisin under high pressure. Innov. Food Sci Emerg Technol 2000; 1(1): 39-47.
44. Ponce E, Pla R, Sendra E, Guamis B and Mor-Mur M. Combined effect of nisin and high hydrostatic pressure on destruction of Listeria innocua and Escherichia coli in liquid whole egg. Int J Food Microbiol 1998; 43(1-2): 15-9.
45. Garriga M, Aymerich MT, Costa S, Monfort JM and Hugas M. Bactericidal synergism through bacteriocins and high pressure in meat model system during storage. Food Microbiol 2002; 19: 509-18.
46. Garcia-Graells C, Masschalck B and Michiels CW. Inactivation of Escherichia coli in milk by high hydrostatic pressure treatment in combination with antimicrobial peptides. J Food Protect 1999; 62(11): 1248-54.
47. Black EP, Kelly AL and Fitzgerald GF. The combined effect of high pressure and nisin on microorganisms in milk. Innovat. Food Sci Emerg Technol 2005; 6(3): 286-92.
48. Schved F, Pierson MD and Juven BJ. Sensitization of E. coli to nisin by maltol and ethyl maltol. Lett Appl Microbiol 1996; 22(3): 189-91.
49. Govaris A, Solomakos N, Pexara A and Chatzopoulou PS. The antimicrobial effect oregano essential oil, nisin and their combination against Salmonella Enteritidis in minced sheep meat during refrigerated storage. Int J Food Microbiol 2010; 137(2-3): 175-80. 50. Terebiznik MR, Jagus RJ, Cerrutti P, De Huergo MS and Pilosof AM. Combined effect of nisin and pulsed electric fields on the inactivation of Escherichia coli. J Food Prot 2000; 63(6): 741-6.
51. Iu J, Mittal GS and Griffiths MW. Reduction in levels of Escherichia coli O157:H7 in apple cider by pulsed electric fields. J Food Prot 2001; 64(7): 964-9.
52. Santi L, Cerrutti P, Pilosof AM and De Huergo MS. Optimization of the conditions for electroporation and the addition nisin for Pseudomonas aeruginosa inhibition. Rev Argent Microbiol 2003; 35(4): 198-204.
53. Liang Z, Mittal GS and Griffiths MW. Inactivation of Salmonella Typhimurium in orange juice containing antimicrobial agents by pulsed electric field. J Food Prot 2002; 65(7): 1081-1087.
54. Long C and Phillips CA. The effect of sodium citrate, sodium lactate and nisin on the survival of Arcobacter butzleri NCTC 12481 on chicken. Food Microbiol 2003; 20: 495- 502.
55. Solomakos N, Govaris A, Koidis P and Botsoglou N. The antimicrobial effect of thyme essential oil, nisin and their combination against Escherichai coli O157:H7 in minced beef during refrigerated storage. Meat Sci 2008; 80(2): 159-66.
56. Carneiro De Melo AMS, Cassar CL and Miles RJ. Trisodium phosphate increases sensitivity of Gram-negative bacteria to lysozyme and nisin. J Food Prot 1998; 61(7): 839–44.
57. Tippayatum P and Chonhenchob V. Antibacterial activities of thymol, eugenol and nisin against some spoilage bacteria. Kasetsart J (Nat Sci) 2007; 41(5): 319-23.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License