IJCRR - 10(6), March, 2018
Pages: 31-36
Date of Publication: 28-Mar-2018
Print Article
Download XML Download PDF
Fulvestrant Efficacy in Artificial Menopausal Hormone Receptor Positive and Human Epidermal Growth Factor Receptor 2 Negative Metastatic Breast Cancer Patients under 50 Years Old
Author: Caglayan Geredeli, Nurgul Yasar
Category: Healthcare
Abstract:Introduction: This study investigated the efficacy of fulvestrant in premenopausal patients with metastatic breast cancer who developed artificial menopause using a luteinizing hormone releasing hormone analogue.
Methods: This retrospective study was conducted at the Istanbul Okmeydani Education and Research Hospital.
Results: A total of 37 patients were evaluated, with a median age of 39 years old (range 27\?49) and a median follow-up time of 20.2 months (0\?78). Of these patients, 86.5% had invasive ductal carcinoma, 5.4% had invasive lobular carcinoma. Bone metastasis was found in 83.8% of the patients, lung metastasis in 21.6%, lymph node metastasis in 16.2%, liver metastasis in 13.5%, and brain metastasis in 5.4%. The progression-free survival (PFS) was a median of 12 months after starting the fulvestrant. The PFS was relatively shorter in those with brain metastases, but there was no statistically significant difference. The median PFS
was 12 months in 2 series and 8 months in 3 and later series, which was statistically significant (p=0.025). The overall survival (OS) was a median of 77 months; it was 86% at 12 months, 63% at 36 months, and 56% at 60 months. The median OS of the 2nd line was 20 months. No grade 3-4 toxic effects were observed.
Conclusion: As in naturally postmenopausal patients, fulvestrant was found to be effective and tolerable in patients treated with artificial menopausal hormone receptor-positive metastatic breast cancer under the age of 50. The fulvestrant was more effective in those who did not previously receive hormonal therapy.
Keywords: Metastatic breast cancer, Premenopausal, Fulvestrant
DOI: 10.7324/IJCRR.2018.1067
Full Text:
Introduction
Endocrine therapy is the preferred form of treatment for hormone receptor (HR) positive early stage and advanced stage breast cancer.(1-3) Endocrine therapy agents that are not cross-resistant to sequential administration prolong the chemotherapy-free period, and they have limited toxicity-effective disease stabilization.(4) Tamoxifen has been the backbone of endocrine therapy for the last 30–40 years(1). In metastatic disease, the response rate has increased to 30% with the use of tamoxifen (1-3). Tamoxifen and its metabolites are linked to the estrogen receptor (ER), and this receptor modulation can cause antagonistic effects, such as estrogenic effects (4). Another group of drugs used in endocrine therapy includes aromatase inhibitors (AIs). In randomized clinical trials, AIs were superior to tamoxifen in the treatment of postmenopausal women (1,3). Fulvestrant, another drug used in endocrine therapy, is an ER antagonist that degrades and blocks ERs (5,6). This causes a decrease in the cellular levels of both ERs and progesterone receptors (PRs) (7, 8). In phase 3 randomized trials, fulvestrant was found to be as effective as anastrozole in postmenopausal women who had previously received endocrine therapy, and it was well tolerated (9, 10).
Fulvestrant can be used in combination with luteinizing hormone-releasing hormone(LHRH) analogues in the anti-estrogenic premenopausal period, although it is indicated in postmenopausal women for advanced disease treatment(7,8). In premenopausal women, the use of aromatase inhibition is ineffective without over-function suppression, which leads to high estrogen levels(9,11). Therefore, premenopausal women need to undergo artificial menopausal treatment by either surgical menopause or medication(9,11). LHRH agonists are as effective in reducing estrogen levels as a surgical oophorectomy(11). Moreover, combining tamoxifen with LHRH agonists is better than over-ablation alone (12). For this reason, tamoxifen in combination with LHRH analogues, aromatase inhibitors, and fulvestrant can be used for premenopausal women in cases of endocrine-responsive metastatic breast cancer(12). The clinical benefit rate of using anastrozole in combination with an LHRH agonist in HR positive metastatic breast cancer was approximately 70% (13).In addition, fulvestrant was found to be as effective as an AI in women with premenopausal metastatic breast cancer who had suppressed hormonal activity (14-16). A median progression-free survival (PFS) of 6 months and median overall survival (OS) of 32 months were found in patients who had previously received endocrine treatment in small-scale studies with an LHRH analogue plus 250 mg of fulvestrant (16). A subgroup analysis of the PALOMA-3 trial found a median PFS of 5.6 months with LHRH plus fulvestrant in patients with premenopausal HR positive metastatic breast cancer who had previously received endocrine therapy (17).
In this retrospective study, we investigated the efficacy and tolerability of fulvestrant in Turkish patients with artificial menopause-enhanced HR positive premenopausal metastatic breast cancer using an LHRH analogue.
Materials and Methods
This was a retrospective single center study conducted at the Istanbul Okmeydani Education and Research Hospital. The medical information was obtained from the archived files of patients with HR positive and HER2 negative metastatic breast cancer treated in the medical oncology clinic. Those patients using fulvestrant who developed metastasis while taking tamoxifen in the adjuvant period, those who were under 50 years old, and those with artificial menopause using an LHRH analogue in the premenopausal period were included in this research. On the basis of the information obtained from the patient files, the LHRH was used analogously, 3.6 mg of goserelin acetate was used every 28 days, and 500 mg of fulvestrant was administered intramuscularly every 28 days (500 mg loading after 14 days from the first dose). The PFS and OS durations were calculated using the date when starting the LHRH analogue and fulvestrant treatment, date of progression, and the date of the last visit from the patient files. The study was approved byLocal Ethics Committee.
Statistical Methods
The Statistical Package for the Social Sciences (SPSS) version 15.0 for Windows (SPSS Inc., Chicago, IL, USA) was used for the statistical analysis. The comparisons of the ratios in the groups were made using a chi-squared analysis. The Monte Carlo simulation was applied when the conditions were not met. The survival analyses were performed with a Kaplan Meier analysis. The statistical significance level of alpha was accepted as p <0.05.
Results
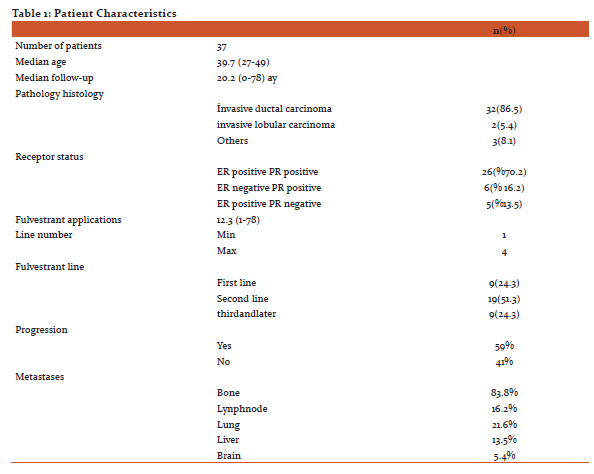
Thirty-seven patients were included in this study, and their mean age was 39.7 (27–49) years old. The median follow-up time was 20.2 (1–78) months. Of the patients, 83.8% had bone metastases, 16.2% had distant lymph node metastases, 21.6% had lung metastases, 13.5% had liver metastases, and 5.4% had brain metastases. Twenty-six(86.5%) of the patients had invasive ductal carcinomas, 2 (5.4%) had invasive lobular carcinomas, and 3 (8.1%) had other histological types. Twenty-six (70.2%) were both ER positive and PR positive, 6 (16.2%) were ER negative and PR positive, and 5 (13.5%) were ER positive and PR negative. Fulvestrant at least the first line was used, thefourth at the most. Moreover, 24.3% of the patients were in the 1stline, 51.3% were in the 2nd line, and 24.3% were in the 3rd line of treatment. A total of 456 fulvestrant applications were performed, and the mean number of treatments was 12.3 (1–78)(Table 1). When considering the number of patients who had previously received endocrine treatments, there were some patients who did not receive endocrine therapy, as well as some patients who received 3 lines of hormonal treatment during the metastatic period.
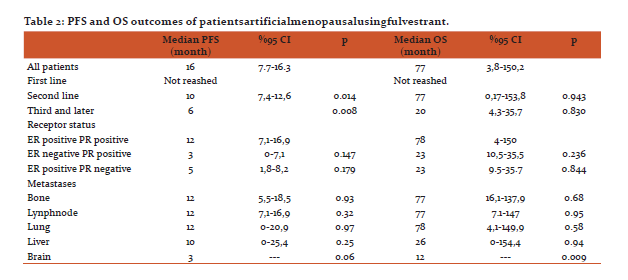
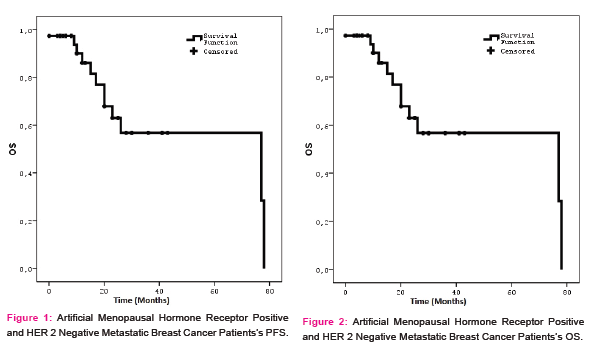
During the follow-up period, 59% of the patients developed progression, 41% had no progression, and they continued to use the LHRH plus fulvestrant. The median PFS was12 (95% confidence interval = 7.7–16.3) months (Figure 1). The 12-month PFS was 51.6%, the 24-month PFS was 32.9%, and the 36-month PFS was 20.5%. The median PFS had still not been reached during the 20.2-month median follow-up in the first line use of fulvestrant in the metastatic period, but the median PFS was 10 months in the second line and 6 months in the third line. There was a statistically significant difference in favor of those who used fulvestrant in the first line when compared to those who used it in the second and third lines (p = 0.014 and p = 0.008, respectively) (Table 2). The use of LHRH plus fulvestrant was more effective in the patients that were both ER positive and PR positive. The median PFS was 12 months in the both receptor positive patients, whereas the median PFS was 3 months (p = 0.179) in the ER negative and PR positive patients, and it was 5 months in the PRnegative and ER positive patients. However, there was no statistical difference (p = 0.147) (Table 2).
The estimated median OS was 77 months after the fulvestrant was started (Figure 2). The estimated median 12-month OS was 86%, the 36-month OS was 63%, and the 60-month OS was 56.7%. The median OS was 12 months in the patients with brain metastases, and the survival was significantly shorter in the patients with other metastatic sites (p = 0.009) (Table 2). The median OS was not reached during the median follow-up period of 20.2 months when the fulvestrant was used as the first line of treatment in those who did not receive endocrine treatment before the fulvestrant in the metastatic period. The median OS was 77 months in the patients who had previously undergone1 line of endocrine therapy, and there was no statistically significant difference (p = 0.830). Moreover, there was no statistically significant difference (p = 0.236) (Table 2), even though the median OS was 23 months, both the ER negative and PR positive group and the PR positive and ER negative group, and the median overall survival was 77 months in the PR positive and ER positive group (Table 2). Finally, toxic effects (such as myalgia, arthralgia, fever, and bone complications) were observed in 38% of the patients, but grade 3–4 toxic effects were not observed.
Discussion
In the prospective multimodal FIRST study, 500 mg of fulvestrant was administered intramuscularly every 28 days in patients with HR positive metastatic breast cancer(18). In addition, 1 mg of anastrozole was compared with the fulvestrant, with a median PFS of 23.4 months and a median OS of 54 months. In the prospective multicenter double-blind phase 3 randomized FALCON study, patients with postmenopausal HR positive metastatic breast cancer who were not previously treated with endocrine therapy were included, and the median PFS was 16.6 months (19). In the study by Bartsch et al., patients with premenopausal HR positive metastatic breast cancer who had already received a very large number of endocrine treatments were treated with 250 mg of fulvestrant, and the median PFS was6 months, while the median OS was 32 months (16). In the study by Bergh et al. (FACT), the median PFS was 10.8 months and the median OS was 37.8 months when using 250 mg of fulvestrant in a premenopausal group who had not received endocrine treatment previously(20). In the study by Loibl et al. (PALOMA-3 subgroup), the median PFS was 5.6 months when using 500 mg of fulvestrant in the treatment of patients with premenopausal metastatic breast cancer who had previously received endocrine treatment(17). In other studies, the median PFS was 5.6–6 months in patients who had previously received multiple endocrine treatments (16,20), while the median PFS was 16.6–23.4 months in patients who had not previously received endocrine treatments(18,19). In our study, the premenopausal (in artificial menopause) hormone receptor-positive patients with metastatic breast cancer who had not previously received endocrine therapy had not reached the median PFS during the20.2-month follow-up period. In the patients who previously received one line of endocrine treatment, the median PFS was 10 months, and in the patients who previously received more than one line of endocrine treatment, the median PFS was 6 months.
In the studies mentioned above, the median OS was 32 months in the patients who had previously received multiple endocrine treatments (16), while the median OS was 37.8–54 months in the patients who had not previously received endocrine treatment(18,19). In our study, the median OS had not yet been reached after 20.2 months of follow-up, and the estimated OS was 77 months. We think that this OS period, which was as long as 77 months, was due to the younger ages of the patients and less comorbidities. Bartsch et al. found a median PFS of 6 months and a median OS of 32 months in endocrine-treated HR positive premenopausal metastatic breast cancer patients with the fulvestrant plus LHRH analogue combination (16). In thePALOMA-3 study, Loibl et al. found a median PFS of 5.6 monthsin endocrine-treated HR positive premenopausal metastatic breast cancer patients with the fulvestrant plus LHRH analogue combination (17). We found a median PFS of 12 months in our study. Moreover, the 12-month median PFS that we found was twice the median PFSs of 5.6 and 6 months in the previous studies. We believe that our PFS was longer because our patient group included patients who have never used endocrine therapy in the metastatic period, and patients who had received fulvestrant in the first line of treatment. In our study, the premenopausal (in artificial menopause) HR positive metastatic breast cancer patients who had not previously received endocrine therapy did not reach the median PFS during the 20.2-month follow-up period. Therefore, we believe that longer survival times can be achieved by using fulvestrant as the first line of treatment in premenopausal HR positive metastatic breast cancer patients.
Based on the results of this study, the endocrine treatment of patients with HR positive metastatic breast cancer who were artificially menopausal with an LHRH analogue using 500 mg of fulvestrant was as effective and tolerable as in postmenopausal patients. Fulvestrant has been shown to be more effective in patients with metastatic breast cancer who have not previously received endocrine therapy in the metastatic period.
Conclusion
As in naturally postmenopausal patients, fulvestrant was found to be effective and tolerable in patients treated with artificial menopausal hormone receptor-positive metastatic breast cancer under the age of 50. The fulvestrant was more effective in those who did not previously receive hormonal therapy.
Acknowledgements
Authors acknowledge the immense help received from the scholars whose articles are cited and included in references of this manuscript. The author is also grateful to authors / editors / publishers of all those articles, journals and books from where the literature for thisarticle has been reviewed and discussed.
Source of funding: No fundingsource
Conflict of interest: No conflict of interest
References:
1. Bonneterre J, Buzdar A, Nabholtz JM, Robertson JF, Thürlimann B, von Euler M, et al. Anastrozole is superiortotamoxifen as first-line therapy in hormone receptor positive advanced beast carcinoma. Cancer 2001;92:2247–58.
2. Bajetta E, Procopio G, Ferrari L, Martinetti A, Zilembo N, Catena L, et al. A randomized multicenter prospective trial assessing long acting release octreotidepamoateplus tamoxifen as a first line therapy for advanced breastcarcinoma. Cancer 2002;94:299–304.
3. Paridaens R, Dirix L, Lohrisch C, Beex L, Nooij M, Cameron D, et al. Mature results of a randomized phase II multicenter study of exemestane versus tamoxifen as first-line hormone therapy for postmenopausal women with metastatic breast cancer. AnnOncol 2003;14:1391–8.
4. Dutertre M, Smith CL. Molecular mechanisms of selective estrogen receptor modulator (SERM) action. J Pharmacol Exp Ther 2000;295:431–7.
5.Wakeling AE, Dukes M, Bowler J. A potent specific püre antiestrogen with clinical potential. CancerRes1991;51:3867–73.
6.Fisher B, Jeong JH, Dignam J, Anderson S, Mamounas E, Wickerham D, et al. Findings from recent National Surgical Adjuvant Breast and Bowel Project adjuvant studies in stage I breastcancer. J Natl Cancer Inst Monogr 2001;30:62–6.
7.Howell A, Osborne CK, Morris C, Wakeling AE. ICI 182,780 (Faslodex): development of a novel, ‘pure’ antiestrogen. Cancer 2000;89:817–25.
8.Robertson JF, Nicholson RI, Bundred NJ, Anderson E, Rayter Z, Dowsett M, et al. Comparison of the short-term biological effects of 7alpha-[9-(4,4,5,5,5- pentafluoropentylsulfinyl)-nonyl]estra-1,3,5, (10)-triene- 3,17 beta-diol (Faslodex) versus tamoxifen in postmenopausal women with primary breast cancer. Cancer Res 2001;61:6739–46.
9.Howell A, Robertson JF, Quaresma Albano J, Aschermannova A, Mauriac L, Kleeberg UR, et al. Fulvestrant, formerly ICI 182,780, is as effective as anastrozole in postmenopausal women with advanced breast cancer progressing after prior endocrine treatment. J Clin Oncol 2002;20:3396–403.
10. Osborne CK, Pippen J, Jones SE, Parker LM, Ellis M, Come S, et al. Double-blind, randomized trial comparing the efficacy and tolerability of fulvestrantversus anastrozole in postmenopausal women with advanced breast cancer progressing on prior endocrine therapy: results of a North American trial. J Clin Oncol 2002;20:3386–95.
11.Taylor CW, Green S, Dalton WS, Martino S, Rector D, Ingle JN, et al. Multicenter randomized clinical trial of goserelinversus surgical ovariectomy in premenopausal patients with receptor-positive metastatic breast cancer: an intergroup study. J Clin Oncol 1998;16:994–9.
12.Klijn JG, Blamey RW, Boccardo F, Tominaga T, Duchateau L. Combined hormone agents trial ists’ group and the european organization for research and treatment of cancer. Combined tamoxifen and luteinizing hormone-releasing hormoneagonists versus LHRH agonist alone in premenopausal advanced breast cancer: a meta-analysis of four randomized trials. J Clin Oncol 2001;19:343–53.
13.Cheung KL, Agrawal A, Folkerd E, Dowsett M, Robertson JF, Winterbottom L., et al. Suppression of ovarianfunction in combinationwith an aromatase inhibitor as treatment for advanced breast cancer in pre-menopausal women. Eur J Cancer 2010;46:2936–42.
14.Robertson JF, Semiglazov V, Nemsadze G, Dzagnidze G, Janjalia M, Nicholson RI, et al. Effects of fulvestrant 250mg in premenopausal women with oestrogen receptor- positiveprimarybreastcancer. Eur J Cancer 2007;43:64–70.
15. O.E. Young, L. Renshaw, E.J. Macaskill, S. White, D. Faratian, J.St.J. Thomas, J.M. Dixon. Effects of fulvestrant 750 mg in premenopausal women with oestrogen-receptor-positive primary breast cancer. Eur J Cancer 2008. 391-399
16.Bartsch R, Bago-Horvath Z, Berghoff A, DeVries C, Pluschnig U, DubskyP,et al.Ovarian function suppression and fulvestrant as endocrinetherapy in premenopausal women with metastatic breast cancer. Eur J Cancer 2012.1932-1938.
17-Loibl S, Turner NC, Ro J, Cristofanilli M, Iwata H, Im SA, et al. Palbociclib Combined with fulvestrant in premenopausal women with advanced breast cancer and prior progression on endocrinetherapy: PALOMA-3 Results. The Oncologist 2017;22:1028–1038
18-Ellis MJ, Llombart-Cussac A, Feltl D, Dewar JA, Jasiówka M, Hewson N, et al. Fulvestrant 500 mg versus Anastrozole 1 mg for the first-line treatment of advanced breast cancer: Overall survival analysis from the phase II F?rst Study. J Clin Oncol 2015.
19-Robertson JFR, Bondarenko IM, Trishkina E, Dvorkin M, Panasci L, Manikhas A, et al. Fulvestrant 500 mg versusanastrozole 1 mg for hormone receptor-positive advanced breast cancer (FALCON): An international randomised double-blind phase 3 trial. Lancet 2016; 388: 2997–3005
20-Bergh J, Jönsson PE, Lidbrink EK, Trudeau M, Eiermann W, Brattström D, et al.FACT: An open-label randomized phase ??? study of fulvestrant and anastrozole in combination compared with anastrozole alone as first-line therapy for patients with receptor-positive postmenopausal breast cancer. J Clin Oncol 2012.
21- Iwata H, Im SA, Masuda N, Im YH, Inoue K, Rai Y,etal.PALOMA-3: Phase III Trial of fulvestrant with or without palbociclib in premenopausal and postmenopausal women with hormone receptor–positive, human epidermal growth factor receptor 2–negative metastatic breast cancer that progressed on prior endocrine therapy—Safety and Efficacy in Asian Patients. J Glob Oncol 2017.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License