IJCRR - 5(19), October, 2013
Pages: 103-109
Date of Publication: 19-Oct-2013
Print Article
Download XML Download PDF
AN INTERVENTIONAL TRIAL TO EVALUATE EFFICACY OF NUTOOL THERAPY IN CONTROL OF PRIMARY INSOMNIA AMONG ELDERLY USING INSOMNIA SEVERITY INDEX
Author: Muhib J., Arish M. K. Shervani, Najeeb J., Kouser F. F., A. Nasir Ansari, Muneeb J.
Category: Healthcare
Abstract:Background and objectives: Insomnia is a unique and complex problem in geriatric population. As many as half of the elderly population between 60 to 79 years of age complain of disturbed sleep, which includes increased sleep latency, decreased quality of sleep, awaking symptoms, excessive daytime sleepiness, mental stress / depression which may result in disturbed intellect, impaired cognition, confusion, psychomotor retardation the whole syndrome of complaints can compromise patient's quality of life and create social and economic burden for caregiver. Considering the wide spread prevalence of insomnia in geriatrics, compounded with lack of wholesome drugs in the treatment, an interventional study was carried out with the objectives to evaluate the efficacy and safety of Nutool therapy in control of geriatric insomnia. Methods: A total of 30 elderly primary insomniacs, diagnosed for primary insomnia by Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, were randomly assigned to test and control groups, comprising 15 patients in each group, respectively. In the test group, Nutool was performed with Roghan e Banafsha (500ml) and Roghan e Gul (500ml), both mixed in equal ratio while Nutool was done with liquid paraffin in control group. The therapy was scheduled on alternate days for one month. The change in the severity of insomnia was evaluated on the basis of scores of Insomnia severity index. Statistical analysis was done using student t-test (paired) for comparison in intra group and t-test (unpaired) for inter-group comparison. Results: Nutool therapy showed statistically significant improvement in all the parameters of Insomnia Severity Index, when pre and post interventional values of the parameters were assessed in intra as well as inter group comparisons. Interpretation and conclusion: This intervention proves the efficacy and safety of Nutool therapy in control of insomnia among elderly.
Keywords: Insomnia, geriatrics, Nutool therapy, Insomnia severity index.
Full Text:
INTRODUCTION
Insomnia is a common complaint throughout the world, and is characterized by difficulty in initiating or maintaining sleep or non-restorative sleep, associated with significant morbidity1. Insomnia in the geriatric patients is commonest among sleep complaints reported by population more than 60 years of age. It is consistently associated with significant reduction in the quality of life, higher risk of depression, and increased use
of health care services3. An epidemiological study reports that individuals with insomnia have a 4.5 folds higher probability of presenting with depression compared with those with normal sleep pattern. In addition, primary insomniacs have an elevated risk of manifesting depression within 3.5 years after onset, even in absence of psychological disturbances4.
Primary insomnia is a specific disorder, defined in the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) as a condition of at least 1 month’s duration, not caused by a medical or psychiatric disorder. In addition, a symptom of insomnia (disturbed sleep initiation or sleep maintenance, or early morning awaking) must present and be associated with complaints of daytime dysfunction.
Pharmacological treatment is the most practical approach to insomnia management; however, adverse events most commonly perceived by the insomniacs include alteration in cognitive function, memory and psychomotor activity, with negative effect on routine daily activities, the so-called hangover is a common manifestation. Moreover, rebound insomnia can occur after abrupt withdrawal of hypnotic therapy. National Institute of Health and Clinical Excellence recommends that doctor should consider using non-drug therapy before starting hypnotic drugs5, as side effects and risks associated with long-term use of the drugs are often major reasons for abetting the patients to discontinue their use despite their perception of continued efficacy4. With these limitations in pharmacotherapy there is a growing interest in non-pharmacological interventions for older adults6. Considering this unconvincing scenario regarding the use of drugs and their side effects, researchers are turning to the nature and the traditional pathies. Unani medicine axiomatically comes to the fore as Seher (Insomnia) has successfully been treated since ancient times without considerable obnoxious side effects on the body.
MATERIAL AND METHODS
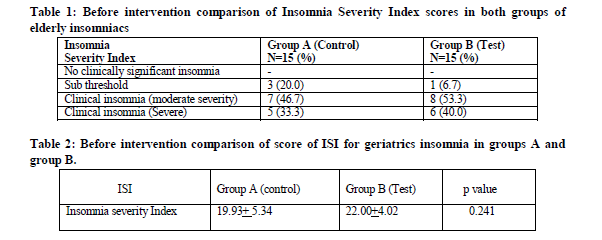
The study was designed as Single blind, randomized, placebo-controlled, concurrent parallel group interventional trial, conducted at National Institute of Unani Medicine Hospital, Bangalore. Ethical clearance was obtained from the Institutional Ethical Committee, NIUM, Bangalore. The study spanned from March 2009 to February 2010. Thirty eligible cases of either sex, above 60 years of age, with primary insomnia and ISI higher than 7, were selected and randomly assigned to Group A (Control Group) and Group B (Test Group), each comprising 15 patients. Group A had been administered liquid paraffin, a placebo drug, under identical conditions as those for test group. Group B was treated with Roghan Banafshan and Roghan gul, mixed in equal quantity of 500 ml each. Efficacy of the drug was evaluated with a Score of Insomnia severity index. Nutool therapy was performed on every alternate day for one month divided in fifteen sittings. Pre and post treatment values of the parameter were assessed statistically.
Patients were advised to observe abstinence from all sorts of hypnotic drugs or measures one week prior to starting the therapy, and no concomitant treatment for insomnia was allowed during the treatment.
Patients were selected on the basis of DSM-IV-TR- Diagnostic criteria for primary insomnia which includes the predominant complaint of difficulty in initiating or maintaining sleep, or non restorative sleep, for at least one month and this sleep disturbance is associated with day time fatigue and may cause clinically significant distress or impairment in social, occupational or other important area of functioning.
Insomniacs associated with secondary medical problems such as Acute fevers or painful conditions, known cases of chronic obstructive pulmonary disease, patients with Obstructive sleep apnea syndrome, Central sleep apnea syndrome, known cases of Restless leg syndrome, Periodic Limb Movement Disorders, Idiopathic Insomnias
or lifelong insomnia, known cases of narcolepsy, sleep disorders associated with diagnosed mental, neurologic and other medical disorders, history of glucocorticoids consumption, known cases of Parkinson, chorea, epilepsy, dementia, Huntington disease, poor mental health, alcohol or drug abuse with in past six months, patients who do not agree to give consent and adhere to protocol were excluded from the study. Ancient Unani physicians used Nutool as an efficient regimen in the treatment of insomnia, as Nutool produces Tarteeb (moistness) in the organ.7, 8
The test drugs, Roghan e banafshan (oil of Viola odorata) and Roghan e gul (oil of Rosa centifolia), used for the Nutool possess properties like Munnawim, Murattib and Muqavvi etc.
Administration of oil: Before starting the therapy, clean gauze was tied just behind the eye brows to avoid spilling of oil over the face. Eyes were protected by keeping a cotton swab soaked in plain water over the closed eye lids. Oil was sterilized and cooled to luke warm to start the therapy. The oil was continuously poured in a rhythmic stream from a distance of half feet 7over the fore head of a patient lying in supine position. The oil flowing down the head was collected in a container placed beneath the outlet of the nutool table. The collected oil was reused to maintain an uninterrupted flow of oil over head for duration of 30 minutes.
Statistical analysis
Results on continuous measurements are presented on Mean ± SD (Min-Max) and results on categorical measurements are presented in Number (%). Significance is assessed at 5 % level of significance, unpaired Student t test ( two tailed, independent) has been used to find the significance of study parameters on continuous scale between two groups (Inter group analysis) and paired Student t test (two tailed, dependent) has been used to find the significance of study parameters on continuous scale within each group.
DISCUSSION
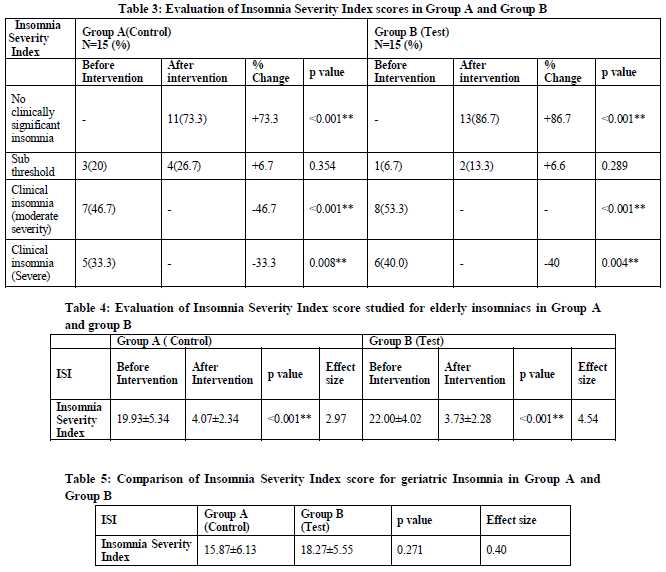
The purpose of test drug in one group and liquid paraffin in other group is to assess the efficacy of Nutool therapy, because Roghan banafshan with Roghan gul being sedative may relieve insomnia and with liquid paraffin, it would not relieve the symptom. But the present study is the first of its kind evaluating the efficacy of procedure of Nutool therapy, as no satisfactory data is available, suggesting Nutool is effective therapy in management of insomnia. This trial had also been studied in a manner of psychological/behavioral studies, multimodal therapies like cognitive and behavioral studies, where studies had been evaluated the pre and post changes and thus p values were observed within group (intra group comparison as opposed to between groups) by comparing p value of two groups (inter group) we had evaluated the variation of therapy in those groups, furthermore, if inter group comparison showed any significant improvement in outcome, this can be considered as the superiority of the test oil over pharmacological inert liquid in improving the variable, sleep latency, mental depression and day time somnolence. Hence pre and post interventional changes within both the groups are evaluated to prove the efficacy of Nutool therapy as a whole.
The Intra group comparisons were made and findings were significant in both the groups with p value<0.001. These changes from base line data for the subjective sleep variable mentioning current sleep pattern, daily functioning (e.g. Day time fatigue, ability to function at work, concentration, mood, memory), impairment of quality of life, current sleep problems, sleep initiation, sleep maintenance and interruptions indicated that Nutool therapy significantly reduces insomnia severity in both the groups as insomnia severity index evaluated insomnia severity, and findings come as an outcome in consonance with the improvement observed in pre and post intervention with p<0.001. This also proves the efficacy of Nutool therapy as a whole. Effect size was 2.97 in group A and effect size of group B as 4.54 with d>1.20 considered as very large effect statistically.
In group A, of 7 patients of moderate severity 6 were relieved to no clinically significant and one patient to sub threshold insomnia. All the 3 patients of sub threshold insomnia were relieved to normal. Among 5 patients having severe insomnia, the severity after treatment decreased to normal in 2 patients and to sub threshold in 3 patients.
In group B, 8 patients of moderate severity 7 were relieved to normal and 1 to sub threshold insomnia. 1 patients of sub threshold insomnia was relieved to normal, and among 6 patients having severe insomnia, the severity after treatment decreased to normal in 5 patients and to sub threshold in 1 patients.
Effect size 2.97 in group A and effect size of group B as 4.54 with d>1.20 considered as very large effect statistically.
However, variation in pre intervention and post intervention values was significant in
both groups (P<0.001). This also proves the efficacy of Nutool therapy, in which any
medicated oil or non medicated liquid if poured have significant effect.
CONCLUSION
- Nutool therapy showed comparable effect in both test and control group after analysis of post interventional values of various parameters of insomnia severity index; it suggests that Nutool therapy irrespective of use of any kind liquid exerts its own effect by the virtue of its sheer streaming effect on the forehead.
- In addition to the inherent effect of Nutool therapy the efficacy was further enhanced by using Roghan e Banafsha and Roghan e Gul.
Nutool therapy can be used as more widely and to a greater effectiveness in the clinical settings to reduce severities and manifestations of various types of insomnia without side effects that are associated with other pharmacological treatments.
Annexure-I
Insomnia Questionnaire for primary insomnia among geriatric population
Part-A
S. NO. ……………… OPD/ IPD NO. ………….
- Name: ………………………………Age /Sex:………
- Religion…
- Marital status : married/ unmarried/divorced/widow/widower
Life style and behavioural pattern:
1. Appetite Good ……… Fair ………. Poor ……………………
2. Diet Veg. ............ Non Veg........ Mixed ……………………
3. Addiction ……..Smoking …….Alcoholism ……Tobacco Chewing …………. Others………
Concurrent Known illness:
High blood pressure Diabetes
Heart disease Lung disease Liver disease
Abnormal heart rhythm Kidney disease
Head trauma or concussion Seizure disorder Immune disorder Kidney disease Thyroid disease Arthritis Stroke
Depression Anxiety/ panic disorder Drug abuse/dependence Alcoholism
Treatment history:
Allopathic /Unani /Ayurvedic / homeopathic……………………………………………
Duration……………………………..Response………………………………......
Physical examination:
Eye redness…yes/no Fatigue………….yes/no
Part –B
Inclusion criteria:
DSM-IV-TR- Diagnostic criteria for primary insomnia,
Exclusion criteria for ruling out secondary Insomnia:
Part-C
Insomnia severity index
- Please rate the current (i.e., last 2 weeks) severity of your insomnia problem(s).
None mild Moderate Severe Very severe
- Difficulty falling asleep 0 1 2 3 4
- Difficulty staying asleep 0 1 2 3 4
- Problem waking up too
Early __ampersandsig
References:
- Roth T, Hajak G, Ustun TB. Consensus for the pharmacological management of insomnia in the new millennium. Int J Clin Practic 2001; 55(1): 42-52.
- Avidan AY. Insomnia in geriatric patients. Clin Cornerstone 2003; 5(3): 51-60
- Belanger L, Belleville G, Morin MC. Management of hypnotic dyscontinuation in chronic insomnia. Sleep Med Clin 2009; 4: 583-592.
- Breslau N, Roth T, Rosenthal L et al. Sleep disturbances and psychiatric disorders: a longitudinal epidemiological study in young adults. Biol psychiatry 1996; 39(6): 411-8)
- Insomnia newer hypnotic drugs. http://www.nice.org.ku/guidance/index.jsp?action=byIDando=11530. cited on 3-4- 2010
- Krishnan P, Hawranik P. Diagnosis and management of geriatric insomnia: A guide for nurse practitioners. Journal of the American Academy of Nurse Practitioners 2008; 20(12):590.
- Ghani MN. Khazainul Advia. New Delhi: Idarae kitabul shifa; YNM: 120-122,741-742, 802,805, 813,814, 999-1003, 1235-1236. 397-398, 1133-1135,
- Ibn sena. Kulliyat e qanoon. (Urdu translation by Kabir uddin.). vol 1,2 New Delhi: Eijaz Publication House; 2003:156-156.
- Khan A. Qarabadein e Azam, Maqzanul mujarribat. New Delhi: Aijaz publications1996: 609.
- Baghdadi IH. Kitabul mukhtarat fil tib. Vol II. New Delhi: CCRUM; 2005: 429-30
- Sleep disorders In: Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Text Revision American Psychiatric Association. Washington, DC; 2000:533-557.
- Buscemi N, Vandermeer B, Friesen C. Manifestations and management of chronic insomnia in adults. Evid Rep Technol Assess (Summ) 2005 ;( 125):1–10.
- Irwin MR, Cole JC, Nicassio PM. Comparative meta-analysis of behavioral interventions for insomnia and their efficacy in middle-aged adults and in older adults 55+ years of age. Health Psychol 2006; 25(1):3–14
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License